Kutztown University Scanning Electron Microscope Lab
A powerful tool for discovering nature's wonders
The Fred and Martha Hafer Scanning Electron Microscope Lab at Kutztown University of Pennsylvania is a place where undergraduate students and faculty perform cutting-edge research and explore our world on a micro- and nano-scale.

Our Zeiss Gemini 300 Field Emission Scanning Electron Microscope (FE-SEM), is a powerful instrument that uses a perfectly straight lightning bolt (electrons) one third of the width of a human hair to see extremely small things.
We can digitally photograph things as small as 20 nanometers wide. For comparison, a single grain of salt is 300,000 nanometers wide!
Our “resolution limit” detects gaps between things 0.8 nanometers apart.
Who uses the lab?
Our electron microscope is used mostly by Kutztown undergraduate science and art students to study dinosaur eggs, pathogenic fungi, insect anatomy, archeological artifacts, growth of polymers and engineered nanoparticles, formation of crystals in ceramic glazes, and the origin of gold deposits in the Earth.
Does the lab collaborate with others?
YES! We like to collaborate with middle- and high school classes. Students from regional schools provide their own samples that they collected to answer some scientific question they are asking. Classes can either visit the lab in person, or they can analyze their samples from their own classrooms by controlling the SEM remotely.
Because we believe in community, we also collaborate on projects with students from other universities and with local industry who lack electron microscopy facilities.
What can we See with the electron microscope?
Our microscope has many types of electron detectors, each of which sees things in different ways:
- Everhart-Thornley Secondary Electron Detector = great for seeing 3-D shapes of tiny surfaces
- In-lens Secondary Electron Detector = best for extremely small (nanometer-scale) imaging
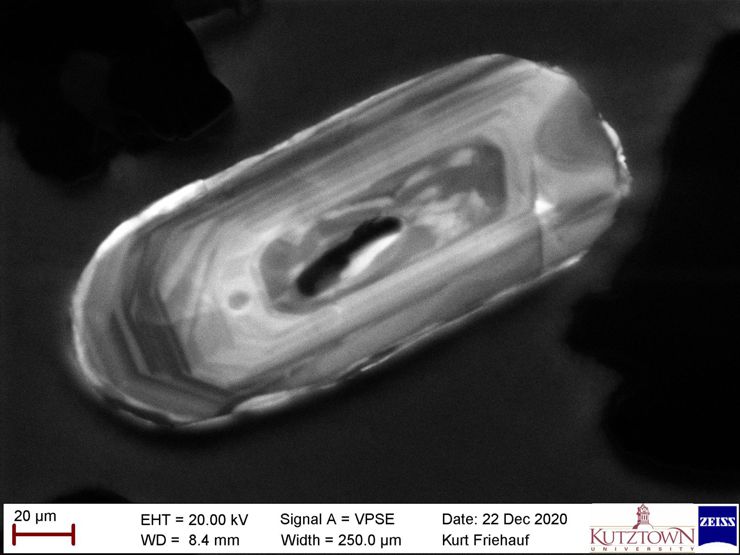
- Variable Pressure Secondary Electron Detector = for analyzing shapes of nonconductive samples without coating
- Backscatter Electron Detector (in chamber) = highlights differences between grains with different chemical compositions
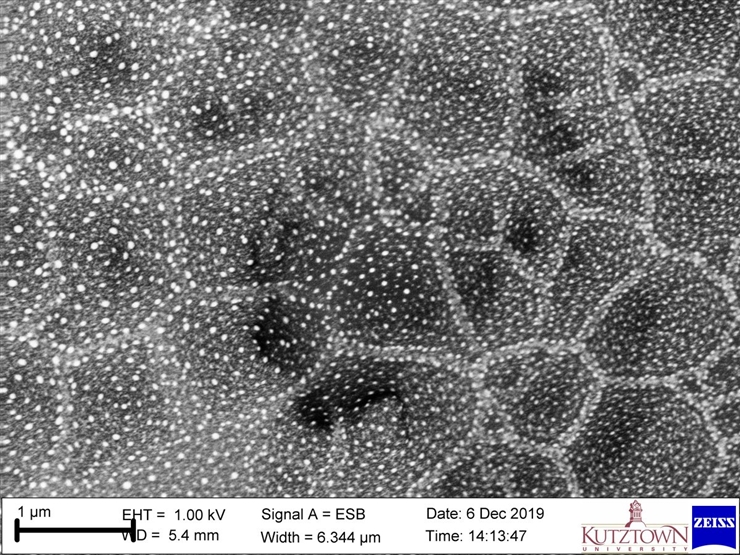
- In-lens Energy-Selective Backscatter Electron Detector = distinguishes chemical compositions for extremely small things (nanometer-scale)
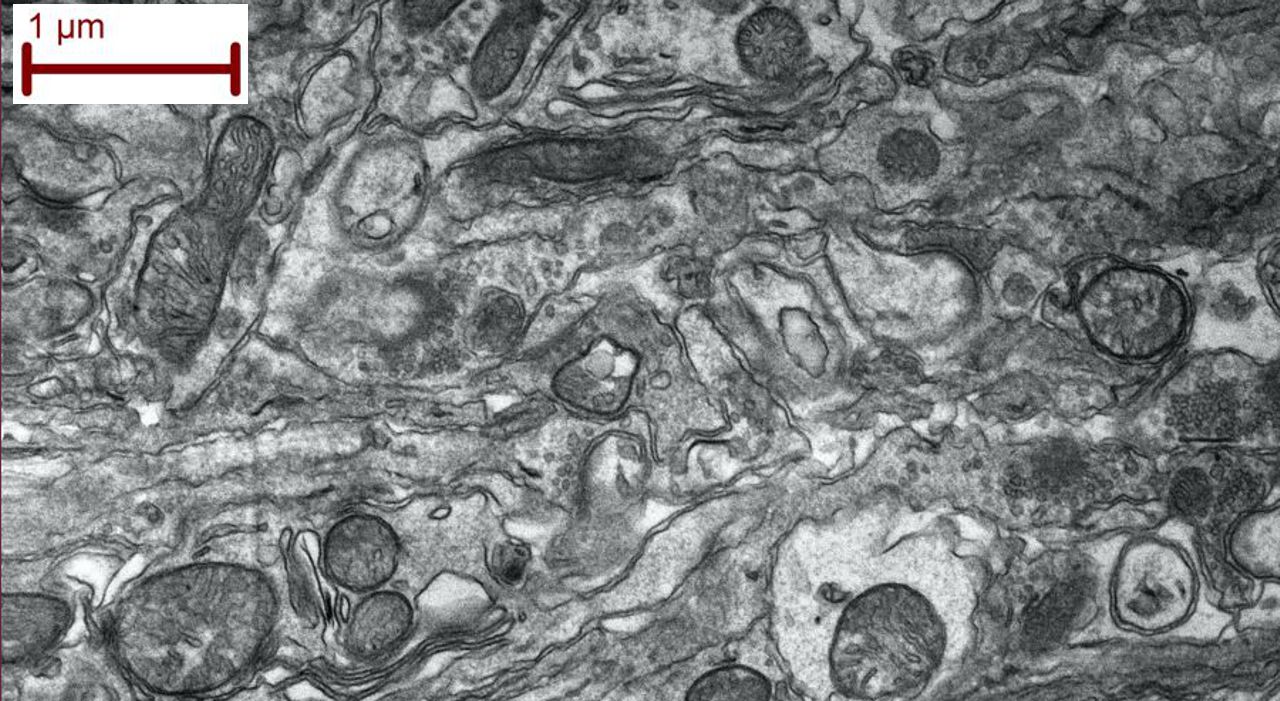
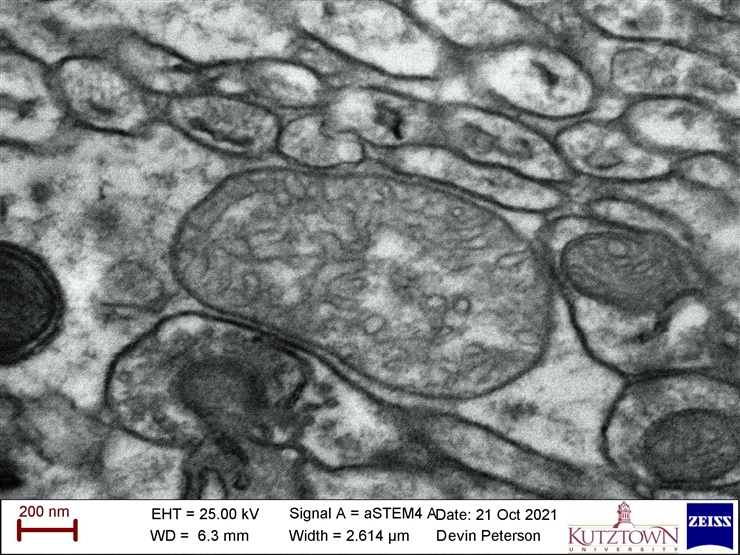
- Scanning Transmitted Electron Detector (STEM) = good for seeing organelles in cells and shapes of ultra-small grains
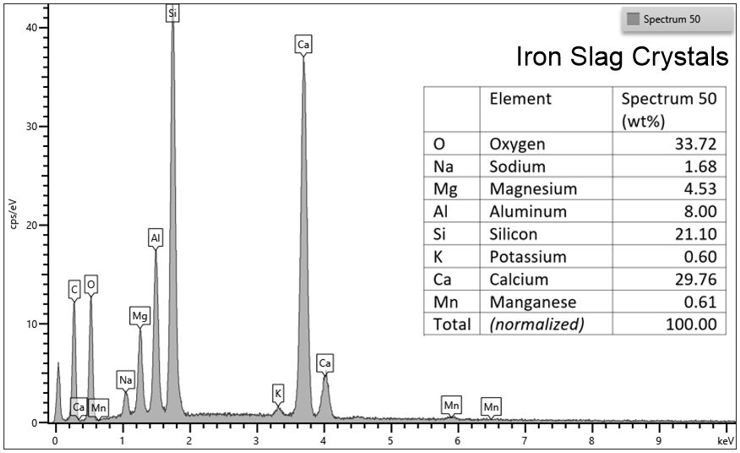
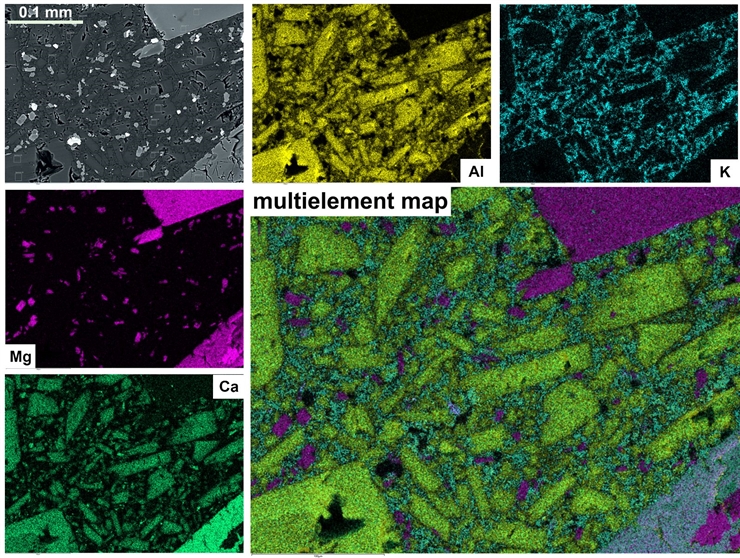
Chemical analytical capabilities
In addition to being able to see the shapes of very tiny things, our SEM is equipped with an Oxford Ultim-Max 100mm2 Energy-dispersive X-ray Spectrometer (EDS) which analyzes the chemical composition of any point on the sample. The instrument can analyze a single one micrometer (0.00004 inch) spot, or do a complete chemical analysis of each individual pixel in an image to create a colorful map that documents broader chemical trends across the sample.