Liquid Chromatography - Mass Spectrometry
Shimadzu 8040 LC-MS/MS
Background
Mass spectrometry is a type of analysis that involves ionization of the molecules you are trying to analyze, then separating those ions according to their mass/charge ratio. Determining the mass of intact molecular ions (or fragments of those molecular ions) leads to a powerful tool for identifying unknown substances.
The mass spectrometers in Kutztown's arsenal are attached to both liquid and gas chromatographs.
Shimadzu Liquid Chromatograph Triple Quadrupole Mass Spectrometer
This instrument has an automated liquid chromatographic (LC) on the front end and a powerful tandem mass spectrometer (MS/MS) as its detector. capable of discriminating molecules according to mass and electrical charge (its mass to charge ratio), and the way molecules dissociate when they collide with inert gas atoms. This detection strategy allows one to single out individual molecules from complex samples, and determine concentrations of molecules at extremely low levels.


In order to analyze a complex mixture with this system, components are first separated on the high-performance liquid chromatograph. Basically the liquid mobile phase is pumped through a column of stationary phase. Samples are injected into that flowing liquid by an autosampler and pushed through the column. As the sample mixture is pushed through the column, the individual components of that mixture are separated before they enter the detector (shown below).
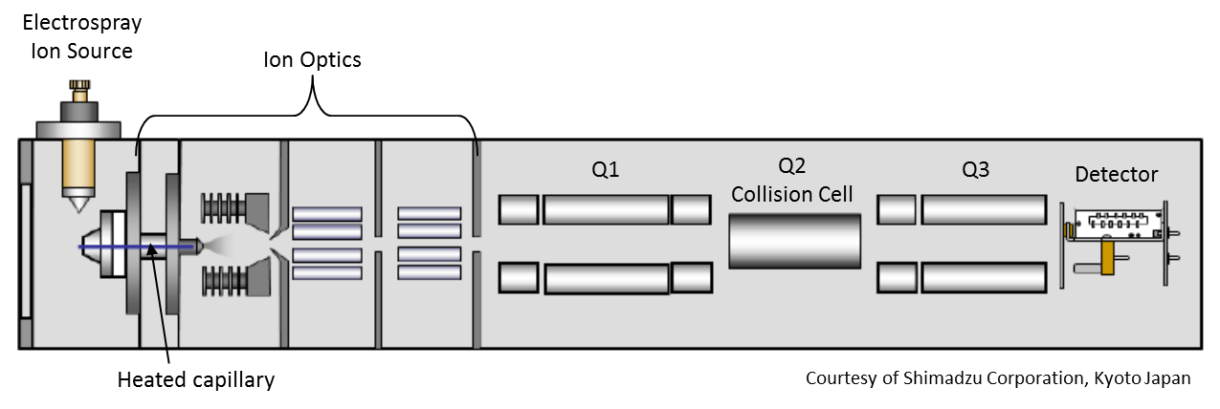

As separated compounds exit the separation column they pass through the electrospray ionization source which can impart positive or negative electrical charges on these molecules. The mobile phase containing the separated molecules passes through an electrically charged nozzle which creates a spray. Some of those fine droplets get swept into a heated tube (desolvation line) to help evaporate remaining liquid. Electrospray is considered a soft ionization source because it does not cause much fragmentation of the original molecules.
The detector includes two mass filters (Q1 and Q3) with a collision cell (Q2) between them. One mode of operating this detector is in the Multiple Reaction Monitoring (MRM) mode.
The first mass filter (Q1) can be set to allow particles with a specific mass to charge ratio to pass into the collision cell (Q2). In the collision cell, those particles (with the correct mass to charge ratio to get past Q1) collide with argon gas atoms and dissociate into smaller fragments. The second mass filter (Q3) allows only a specific fragment to make it to the detector (again based on mass to charge ratio).
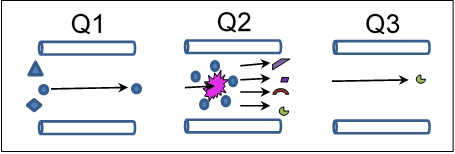
The beauty of this detection scheme is that each molecule that is detected must pass through two screenings before it can register a signal, allowing the detector to be extremely selective and sensitive.
Applications
Recently, we have been using this technique to assess pesticide residues in fruits, honey, bees, and wax, to determine the exposure of bats to pesticides by screening guano, and to quantify drugs in wastewater. The pesticide analysis usually involves extracting the pesticides from the sample into a solvent that can be injected into the instrument.
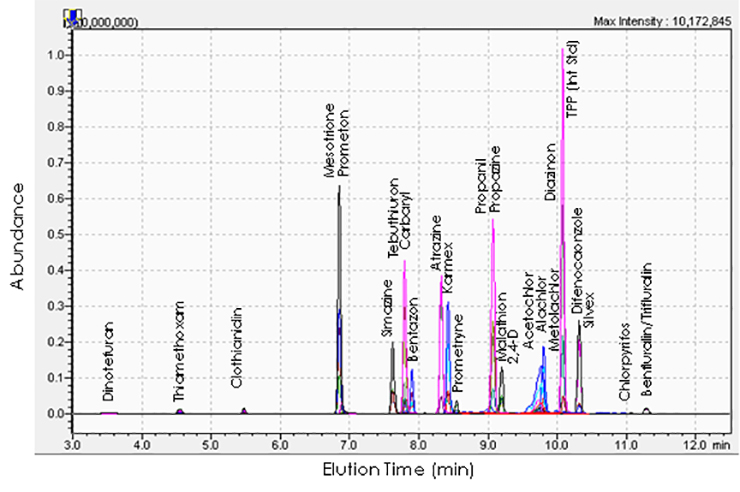

Method development involves finding a chromatography column and mobile phase combination that separates most of the molecules of interest before they reach the detector. Then detector conditions are optimized for each analyte. This includes determining the correct charge on the electrospray, what mass to charge ratios to set for both mass filters, and the optimal collision energy for the collision cell. Once all of these settings are determined the method must be assessed for accuracy before applying it to a particular study.






