Ultraviolet/Visible Spectroscopy
Introduction to UV-Vis Spectroscopy
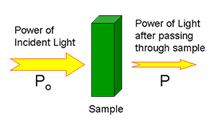
In UV-Vis spectroscopy, a beam of ultraviolet or visible light is directed through a sample. Some of the light may be transmitted through the sample. Light that was not transmitted through the sample was absorbed.
Transmittance (T) is defined as the ratio of P/Po.
Absorbance (A) is defined as -log(P/Po).

A molecule can absorb light by promoting electrons to higher energy levels. The energy of the light being absorbed must match the energy required to promote the electron. Therefore, some wavelengths of light are absorbed by a sample (those wavelengths that match a transition) while others are not. By measuring absorbance as we scan across a wavelength range, a spectrum is obtained that reflects those energetic transitions.
Changes in a chemical system can lead to changes in the way that system absorbs light. This provides a window to analyze these changes.
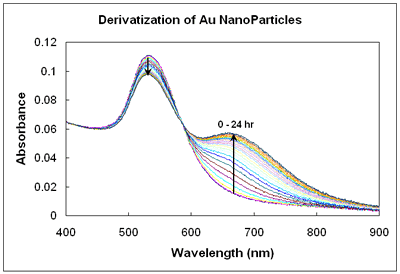
The UV-Vis absorbance spectrum shown here is of gold nanoparticles reacting with a thiol over 24 hours. Changes in the absorbance spectrum over time help decipher the kinetics of the thiol reacting with the surface of the gold nanoparticles.
UV-Vis Instrumentation
Agilent Cary 60
The Cary 60 design is simple, yet elegant. Its fast-scanning monochromator collects spectra in seconds, and the Xe flash lamp is pulsed to avoid sample degradation and interference from room light. The Cary 60 is controlled by intuitive software which allows students who are unfamiliar with spectrophotometers to begin collecting data without extensive software training.
The sample chamber is temperature-controlled by a Peltier heater/cooler to rapidly change sample temperature between 0 to 100 degrees Celsius. Temperature probes are integrated into the system to accurately determine the temperature of the sample inside the cuvette. A stopped-flow accessory is available to measure the kinetics of fast reactions.


Varian Cary 50
We have two older Varian Cary 50 spectrophotometers (before Agilent bought out Varian) which are in perfect working condition. Because of the importance of absorbance spectroscopy in a wide range of disciplines, there are sometimes a lot of people trying to measure absorbance. It is nice to be able to get access to an instrument when you need it.
BioTek Synergy 2 Plate Reader
The BioTek Synergy 2 Plate Reader is capable of measuring absorbance or fluorescence of many samples in a fast, automated fashion. The open drawer (on the left side of this image) accommodates a well plate. We typically use plates with 96 separate compartments. The benefits of this system are that it requires small volumes of sample, and it is possible to analyze up to 96 samples at a time. This instrument is used primarily in biochemistry labs.



Thermo Spectronic Genesys 10 UV Spectrophotometers
Kutztown's fleet of 16 Genesys 10 UV-Vis spectrophotometers is used in a variety of laboratory courses throughout the chemistry and biochemistry curricula. These UV-Vis spectrophotometers are rugged, portable, user-friendly, and are great for quantitative work and kinetics at a single wavelength.
Spectronic 20 Spectrophotometers
Spec 20s have been around since the 1960s, and we have a few that have been around that long. These spectrometers are rugged, rudimentary, visible spectrometers. It is because of these qualities that it is not surprising to see Spec 20s still in use in both educational and industrial labs. The 8 Spec 20s in Kutztown's labs are all operational. Students have the opportunity to take them apart to learn the fundamentals of spectroscopy and instrument design. A box of spare vacuum tubes, light sources, and detectors to is used to keep them going well into the next decade.

A Few Applications of UV-Vis Molecular Absorbance Spectroscopy
Quantitative analysis of all molecules that absorb light
Disruption of DNA double helix
Molecular temperature probes
Determination of equilibrium constants
Enzyme Kinetics
Analysis of mixtures of absorbing species
UV-VIS absorption as a diagnostic for NO in rocket plumes
Analysis of foods
Monitor ozone depletion
Determination of micelle structure
Drug dissolution
Spectral analysis of paintings
Art restoration/color matching
Light-harvesting for solar energy
Determining wavelength response of fish eyes - Absorption spectra of retinal pigments






