Atomic Spectroscopy
Background
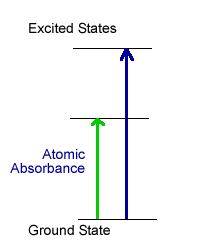
Atomic Spectroscopy is based upon the ability of atoms to absorb or emit light. In atomic absorption spectrophotometry (see graph at right), the atoms are heated enough in a flame or graphite tube to free them from solvents and disrupt the formation of salts, but not enough to pump electrons to an excited electronic state. The free atoms, with electrons in the ground state, are ready to absorb light of just the right energy to promote electrons to an excited electronic state. The greater the concentration of that atom in the sample, the less light reaches the detector; so it is relatively straightforward to determine concentration of a particular atom in a sample by measuring how much light it absorbs.
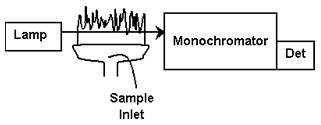
Instrumentation used to carry out atomic absorption spectrophotometry requires a source of light that matches the narrow bands of light that a particular atom absorbs (a hollow cathode lamp), a flame or graphite furnace to heat the sample, a monochromator to select the wavelength of light, and a photodetector.
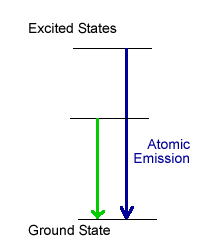
In atomic emission spectrophotometry (see graph at right), the purpose of heating the sample is not only to free the atoms from the solvent and formation of salts, but to provide enough energy to pump electrons into excited electronic energy levels. As these electrons fall back to lower energy levels, they emit light at specific wavelengths. The more light that is detected at that wavelength, the more of that element must be in the sample. Instrumentation to carry out atomic emission would not need a lamp. Inductively coupled plasmas exist at extremely high temperatures (around the temperature of the surface of the sun), and are used in atomic emission spectroscopy.
Atomic Spectroscopy Instrumentation
Varian (now Agilent) 220 FS Atomic Spectrophotometer
This Atomic Spectrophotometer operates in both atomic absorption and emission modes. The FS in the name stands for Fast Scanning, which means that this instrument can take a measurement for one element, then quickly scan to the next wavelength to collect a measurement for another element, then the next, etc. This means that the instrument can collect data on approximately ten elements in the same time that it would take to measure one element. It has 4 lamp sockets, and can automatically switch between these four lamps using a moveable mirror. Automation of the mirror position, monochromator wavelength, and data collection are can be fully automated.

We can remove the flame burner head, and replace it with a graphite furnace. The graphite furnace is important because the atoms remain in the path of light about a thousand times longer than in the flame, thus providing a greater opportunity for them to absorb light. The result is that you can detect much smaller amounts for most elements. For example, the EPA safe level for lead in drinking water is 25 parts per billion. Using a graphite furnace, one can readily detect 50 ppb Pb. Using a flame, it is not possible to detect lead at concentrations that low. An autosampler is used with the graphite furnace to allow reproducible sample injection, and injection of up to 50 samples as well as automate the preparation of standards.

Perkin Elmer 3110 Atomic Spectrometer
The PE 3110 is a stand-alone AA that is not interfaced to a PC. The instrument's straight-forward design and rock-solid optical bench leads to stable, reproducible results, and provides a great tool for learning this technique. This spectrometer is in excellent working condition and provides us with flexibility when several groups of students are working on AA at the same time.

Applications of Atomic Absorption and Emission Spectrophotometry
In Analytical Chemistry courses, students are asked to propose an analysis using atomic spectroscopy and execute it. Some of the analyses performed over the past few years have included:
Determination of Mn and Fe in cat hair and cat food
Analysis of Ni and Cu in tea leaves
Determination of Ca and Mg in coal and coal ash
Analysis of a bullet from an antique anti-vampire kit to determine the purity of silver
Determination of the elemental composition of a statue
Antimony and silver in bullet lead
Quantitation of Fe in a few green vegetables
Water hardness
Effectiveness of soils as metal traps
Analysis of lead in paint
Determination of zinc, sodium and potassium in urine
Analysis of soil for Zn around a superfund site






